|
|
|
NK cells are cytotoxic; small granules in their cytoplasm contain proteins such as perforin and proteases known as granzymes. Upon release in close proximity to a cell slated for killing, perforin forms pores in the cell membrane of the target cell, creating an aqueous channel through which the granzymes and associated molecules can enter, inducing either apoptosis or osmotic cell lysis. The distinction between apoptosis and cell lysis is important in immunology: lysing a virus-infected cell could potentially only release the virions, whereas apoptosis leads to destruction of the virus inside. α-defensins, antimicrobial molecules, are also secreted by NK cells, and directly kill bacteria by disrupting their cell walls in a manner analogous to that of neutrophils.[3]
 |
| Antibody-dependent cell-mediated cytotoxicity |
Infected cells are routinely opsonized with antibodies for detection by immune cells. Antibodies that bind to antigens can be recognised by FcϒRIII (CD16) receptors expressed on NK cells, resulting in NK activation, release of cytolytic granules and consequent cell apoptosis. This is a major killing mechanism of some monoclonal antibodies like rituximab (Rituxan), ofatumumab (Azzera), and others. The contribution of antibody-dependent cell-mediated cytotoxicity to tumor cell killing can be measured with a specific test that uses NK-92 that has been transfected with a high-affinity FcR. Results are compared to the "wild type" NK-92 that does not express the FcR.[9]
Cytokines play a crucial role in NK cell activation. As these are stress molecules released by cells upon viral infection, they serve to signal to the NK cell the presence of viral pathogens in the affected area. Cytokines involved in NK activation include IL-12, IL-15, IL-18, IL-2, and CCL5. NK cells are activated in response to interferons or macrophage-derived cytokines. They serve to contain viral infections while the adaptive immune response generates antigen-specific cytotoxic T cells that can clear the infection. NK cells work to control viral infections by secreting IFNγ and TNFα. IFNγ activates macrophages for phagocytosis and lysis, and TNFα acts to promote direct NK tumor cell killing. Patients deficient in NK cells prove to be highly susceptible to early phases of herpes virus infection.
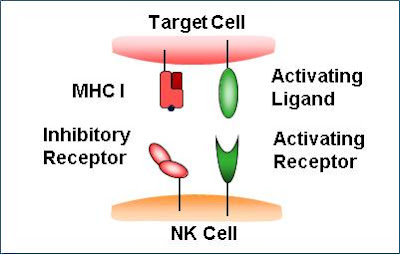
For NK cells to defend the body against viruses and other pathogens, they require mechanisms that enable the determination of whether a cell is infected or not. The exact mechanisms remain the subject of current investigation, but recognition of an "altered self" state is thought to be involved. To control their cytotoxic activity, NK cells possess two types of surface receptors: activating receptors and inhibitory receptors, including killer-cell immunoglobulin-like receptors. Most of these receptors are not unique to NK cells and can be present in some T cell subsets, as well.
The inhibitory receptors recognize MHC class I alleles, which could explain why NK cells preferentially kill cells that possess low levels of MHC class I molecules. This mode of NK cell target interaction is known as "missing-self recognition", a term coined by Klas Kärre and co-workers in the late 90s. MHC class I molecules are the main mechanism by which cells display viral or tumor antigens to cytotoxic T cells. A common evolutionary adaptation to this is seen in both intracellular microbes and tumors: the chronic down-regulation of MHC I molecules, which makes affected cells invisible to T cells, allowing them to evade T cell-mediated immunity. NK cells apparently evolved as an evolutionary response to this adaptation (the loss of the MHC eliminates CD4/CD8 action, so another immune cell evolved to fulfill the function).[10]
 |
| Tumor cell surveillance |
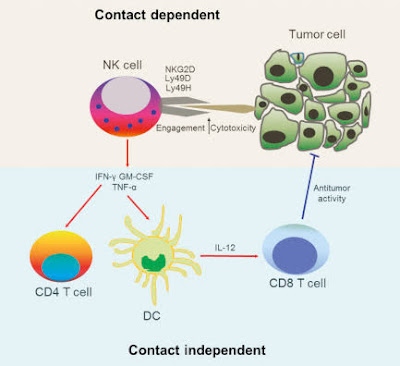
Natural killer cells often lack antigen-specific cell surface receptors, so are part of innate immunity, i.e. able to react immediately with no prior exposure to the pathogen. In both mice and humans, NKs can be seen to play a role in tumor immunosurveillance by directly inducing the death of tumor cells (NKs act as cytolytic effector lymphocytes), even in the absence of surface adhesion molecules and antigenic peptides. This role of NK cells is critical to immune success particularly because T cells are unable to recognize pathogens in the absence of surface antigens.[1] Tumor cell detection results in activation of NK cells and consequent cytokine production and release.
If tumor cells do not cause inflammation, they will also be regarded as self and will not induce a T cell response. A number of cytokines are produced by NKs, including tumor necrosis factor α (TNFα), IFNγ, and interleukin (IL-10). TNFα and IL-10 act as proinflammatory and immunosuppressors, respectively. The activation of NK cells and subsequent production of cytolytic effector cells impacts macrophages, dendritic cells, and neutrophils, which subsequently enables antigen-specific T and B cell responses. Instead of acting via antigen-specific receptors, lysis of tumor cells by NK cells is mediated by alternative receptors, including NKG2D, NKp44, NKp46, NKp30, and DNAM.NKG2D is a disulfide-linked homodimer which recognizes a number of ligands, including ULBP and MICA, which are typically expressed on tumor cells. The role of dendritic cell—NK cell interface in immunobiology have been studied and defined as critical for the comprehension of the complex immune system.[citation needed]
NK cells, along with macrophages and several other cell types, express the Fc receptor (FcR) molecule (FC-gamma-RIII = CD16), an activating biochemical receptor that binds the Fc portion of IgG class antibodies. This allows NK cells to target cells against which a humoral response has been gone through and to lyse cells through antibody-dependant cytotoxicity (ADCC). This response depends on the affinity of the Fc receptor expressed on NK cells, which can have high, intermediate, and low affinity for the Fc portion of the antibody. This affinity is determined by the amino acid in position 158 of the protein, which can be phenylalanine (F allele) or valine (V allele). Individuals with high-affinity FcRgammRIII (158 V/V allele) respond better to antibody therapy. This has been shown for lymphoma patients who received the antibody Rituxan. Patients who express the 158 V/V allele had a better antitumor response. Only 15–25% of the population expresses the 158 V/V allele. To determine the ADCC contribution of monoclonal antibodies, NK-92 cells (a "pure" NK cell line) has been transfected with the gene for the high-affinity FcR.
Adaptive features of NK cells—"memory-like", "adaptive" and memory NK cells
The ability to generate memory cells following a primary infection and the consequent rapid immune activation and response to succeeding infections by the same antigen is fundamental to the role that T and B cells play in the adaptive immune response. For many years, NK cells have been considered to be a part of the innate immune system. However, recently increasing evidence suggests that NK cells can display several features that are usually attributed to adaptive immune cells (e.g. T cell responses) such as dynamic expansion and contraction of subsets, increased longevity and a form of immunological memory, characterized by a more potent response upon secondary challenge with the same antigen. In mice, the majority of research was carried out with murine cytomegalovirus (MCMV) and in models of hapten-hypersensitivity reactions. Especially, in the MCMV model, protective memory functions of MCMV-induced NK cells were discovered and direct recognition of the MCMV-ligand m157 by the receptor Ly49 was demonstrated to be crucial for the generation of adaptive NK cell responses.In humans, most studies have focused on the expansion of an NK cell subset carrying the activating receptor NKG2C (KLRC2). Such expansions were observed primarily in response to human cytomegalovirus (HCMV),[14] but also in other infections including Hantavirus, Chikungunya virus, HIV, or viral hepatitis. However, whether these virus infections trigger the expansion of adaptive NKG2C+ NK cells or whether other infections result in re-activation of latent HCMV (as suggested for hepatitis ), remains a field of study. Notably, recent research suggests that adaptive NK cells can use the activating receptor NKG2C (KLRC2) to directly bind to human cytomegalovirus-derived peptide antigens and respond to peptide recognition with activation, expansion, and differentiation,a mechanism of responding to virus infections that was previously only known for T cells of the adaptive immune system.
As the majority of pregnancies involve two parents who are not tissue-matched, successful pregnancy requires the mother's immune system to be suppressed. NK cells are thought to be an important cell type in this process.These cells are known as "uterine NK cells" (uNK cells) and they differ from peripheral NK cells. They are in the CD56bright NK cell subset, potent at cytokine secretion, but with low cytotoxic ability and relatively similar to peripheral CD56bright NK cells, with a slightly different receptor profile.These uNK cells are the most abundant leukocytes present in utero in early pregnancy, representing about 70% of leukocytes here, but from where they originate remains controversial.
These NK cells have the ability to elicit cell cytotoxicity in vitro, but at a lower level than peripheral NK cells, despite containing perforin. Lack of cytotoxicity in vivo may be due to the presence of ligands for their inhibitory receptors. Trophoblast cells downregulate HLA-A and HLA-B to defend against cytotoxic T cell-mediated death. This would normally trigger NK cells by missing self recognition; however, these cells survive. The selective retention of HLA-E (which is a ligand for NK cell inhibitory receptor NKG2A) and HLA-G(which is a ligand for NK cell inhibitory receptor KIR2DL4) by the trophoblast is thought to defend it against NK cell-mediated death.
Uterine NK cells have shown no significant difference in women with recurrent miscarriage compared with controls. However, higher peripheral NK cell percentages occur in women with recurrent miscarriages than in control groups.
NK cells secrete a high level of cytokines which help mediate their function. NK cells interact with HLA-C to produce cytokines necessary for trophoblastic proliferation. Some important cytokines they secrete include TNF-α, IL-10, IFN-γ, GM-CSF and TGF-β, among others. For example, IFN-γ dilates and thins the walls of maternal spiral arteries to enhance blood flow to the implantation site.
By shedding decoy NKG2D soluble ligands, tumor cells may avoid immune responses. These soluble NKG2D ligands bind to NK cell NKG2D receptors, activating a false NK response and consequently creating competition for the receptor site. This method of evasion occurs in prostate cancer. In addition, prostate cancer tumors can evade CD8 cell recognition due to their ability to downregulate expression of MHC class 1 molecules. This example of immune evasion actually highlights NK cells' importance in tumor surveillance and response, as CD8 cells can consequently only act on tumor cells in response to NK-initiated cytokine production (adaptive immune response).