|
| Lymphocytes: Definition, Ranges,Count & Functions |
Lymphocytes are one of several different types of white blood cells. Each type of white blood cell has a specific function, and they all work together to fight illness and disease.
White blood cells are an important part of your immune system. They help your body fight antigens, which are bacteria, viruses, and other toxins that make you sick. If your doctor says you have a weakened immune system, that means there aren’t enough white blood cells in your bloodstream.
 |
| Lymphocytes: Definition, Ranges,Count & Functions |
Lymphocyte levels can change according to a person’s race, gender, location, and lifestyle habits.
The normal lymphocyte range in adults is between 1,000 and 4,800 lymphocytes in 1 microliter (µL) of blood. In children, the normal range is between 3,000 and 9,500 lymphocytes in 1 µL of blood.
Unusually high or low lymphocyte counts can be a sign of disease.
 |
| Lymphocytes: Definition, Ranges,Count & Functions |
Lymphocyte Counts
Lymphocytes are a component of complete blood count (CBC) tests that include a white blood cell differential, in which the levels of the major types of white blood cells are measured. Such tests are used to assist in the detection, diagnosis, and monitoring of various medical conditions. Lymphocyte counts that are below the reference range, which varies for adults and children, may be indicative of lymphocytopenia (lymphopenia), whereas those above it are a sign of lymphocytosis. Lymphocytopenia is associated with a variety of conditions, ranging from malnutrition to rare inherited disorders such as ataxia-telangiectasia or severe combined immunodeficiency syndrome. Lymphocytosis typically is associated with infections, such as mononucleosis or whooping cough, certain cancers of the blood or lymphatic system such as multiple myeloma and chronic lymphocytic leukemia, and autoimmune disorders that cause chronic inflammation, such as inflammatory bowel disease.
A low lymphocyte count, called lymphocytopenia, usually occurs because:
- your body isn’t producing enough lymphocytes
- lymphocytes are being destroyed
- lymphocytes are trapped in your spleen or lymph nodes
Lymphocytopenia can point to a number of conditions and diseases. Some, like the flu or mild infections, aren’t serious for most people. But a low lymphocyte count puts you at greater risk of infection.
Other conditions that can cause lymphocytopenia include:
- undernutrition
- HIV and AIDS
- influenza
- autoimmune conditions, such as lupus
- some cancers, including lymphocytic anemia, lymphoma, and Hodgkin disease
- steroid use
- radiation therapy
- certain drugs, including chemotherapy drugs
- some inherited disorders, such as Wiskott-Aldrich syndrome and DiGeorge syndrome
Lymphocytosis, or a high lymphocyte count, is common if you’ve had an infection. High lymphocyte levels that persist may point to a more serious illness or disease, such as:
- viral infections, including measles, mumps, and mononucleosis
- adenovirus
- hepatitis
- influenza
- tuberculosis
- toxoplasmosis
- cytomegalovirus
- brucellosis
- vasculitis
- acute lymphocytic leukemia
- chronic lymphocytic leukemia
- HIV and AIDS
 |
| Lymphocytes: Definition, Ranges,Count & Functions |
There are different types of B cells and T cells that have specific roles in the body and the immune system.
B cells
Memory B cells circulate in the body to start a fast antibody response when they find a foreign substance. They remain in the body for decades and become memory cells, which remember previously found antigens and help the immune system respond faster to future attacks.
Regulatory B cells or Bregs make up around 0.5 percent of all B cells in healthy people. Although few in number, they have a vital role to play.
Bregs have protective anti-inflammatory effects in the body and stop lymphocytes that cause inflammation. They also interact with several other immune cells and promote the production of regulatory T cells or Tregs.
T cells
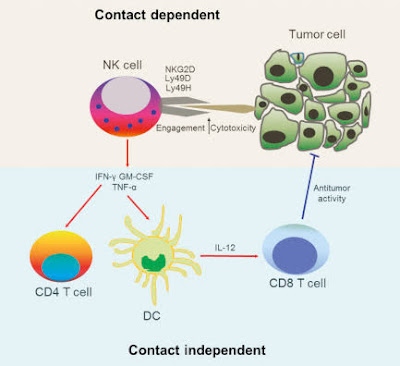
Killer or cytotoxic T cells scan the surface of cells in the body to see if they have become infected with germs, or if they have turned cancerous. If so, they kill these cells.
Helper T cells “help” other cells in the immune system to start and control the immune response against foreign substances.
There are different types of helper T cells, and some are more effective than others against different types of germs.
For instance, a Th1 cell is more effective against germs that cause infection inside other cells, such as bacteria and viruses, while a Th2 cell is more effective against germs that cause infection outside of cells, such as certain bacteria and parasites.
Tregs control or suppress other cells in the immune system. They have both helpful and harmful effects.
They maintain tolerance to germs, prevent autoimmune diseases, and limit inflammatory diseases. But they can also suppress the immune system from doing its job against certain antigens and tumors.
Memory T cells protect the body against previously found antigens. They live for a long time after an infection is over, helping the immune system to remember previous infections.
If the same germ enters the body a second time, memory T cells remember it and quickly multiply, helping the body to fight it more quickly.
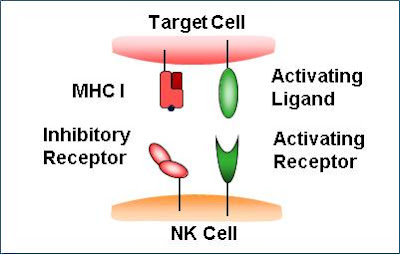
Natural killer T cells are a mixed group of T cells that share characteristics of both T cells and natural killer cells. They can influence other immune cells and control immune responses against substances in the body that trigger an immune response.